Proper liming is essential to derive maximum benefits from fertilizer applications.
Liming is the practice of applying an agent to reduce soil acidity (raise pH) and make soils more favorable for turfgrass growth. Raising soil pH requires a quantity of liming material that is determined by the degree of soil acidity as well as the quality and type of liming material. Soil acidity is determined by a soil test, however, not all soil tests provide accurate information on how much lime should be applied. Most university and commercial laboratories will provide sound recommendations of how much lime needs to be applied to turfgrass areas.
Why is liming important?
Soil pH affects turfgrass health by influencing availability of plant nutrients and other elements, thatch decomposition, some turfgrass pests, and pesticide activity.
Strongly acid soils (pH less than or equal to 5.5) may lead to deficiencies in calcium, magnesium, or phosphorus and increase availability of aluminum and manganese in amounts that may be toxic to turfgrasses. Liming improves plant nutrient availability and reduces toxicity problems in acid soils.
In strongly alkaline soils (pH greater than or equal to 8.5), the formation of insoluble tricalcium phosphate makes the phosphorus unavailable to the plant. Iron chlorosis, an indication of iron deficiency, may be seen on some plants growing in soils high in pH. Since most soils in Pennsylvania are not strongly alkaline, these types of deficiencies are not often encountered. Exceptions may occur when too much lime is applied to established turf or to the soil prior to planting.
Many beneficial soil microorganisms do not thrive in strongly acid soils. Some of these microorganisms break-down certain nitrogen fertilizers, thereby releasing the nitrogen for use by the turfgrass. Fertilizers containing nitrogen from ureaform, sulfur-coated urea, or natural organic sources are not effective unless certain microorganisms are present in sufficient quantities.
Soil microorganisms also aid in the decomposition of thatch and grass clippings. Thatch is the dense accumulation of organic material on the soil surface beneath the grass. A thatch layer restricts movement of air, water, nutrients, and pesticides into the soil. Soil pH in the range of 6.0 to 7.0 increases microbial activity and helps reduce thatch.
Some turfgrass diseases are influenced by soil pH. Although the reasons for this are not well understood, there is some evidence to suggest that in very acid soils the populations of microorganisms that suppress pathogenic fungi are reduced. In addition, plants growing in acid soils may be more susceptible to disease because they are suffering from nutrient deficiencies or aluminum toxicity. Conversely, there are at least two turfgrass diseases (take-all patch and Fusarium patch) that are suppressed in acid soils. Fortunately, these diseases rarely cause problems in home lawns. Optimum pH (6.0 to 7.0) does not prevent turfgrass disease, but it can reduce the severity of infestation.
Acidic soils create conditions that favor growth of certain weed species. One of the most common and difficult-to-control weeds, moss, in more prevalent in moderately to strongly acidic soils than in neutral soils or slightly acid soils. Shepherds purse is a lawn weed that is a good indicator of moderately to strongly acidic soils. Although weeds cannot be controlled with lime applications, applying lime before soils become too acid is one means of preventing severe weed infestation.
Research has shown that the activity of some pesticides is influenced by soil pH. Strongly acid soils can reduce the effectiveness of some turfgrass herbicides and insecticides.
Why do soils become acid?
Soils become acid through natural processes and human activities. The pH of most soils is controlled by the amount of rainfall. In humid areas, such as the northeastern United States, rainfall percolates through the soil, leaching ions such as calcium and magnesium which prevent the soil from becoming more acid and replacing them with acidic ions such as hydrogen and aluminum. Other natural processes that increase soil acidity include root growth and decay of organic matter by soil microorganisms.
Human activities that increase soil acidity include fertilization with ammonium-containing fertilizers and pollution of industrial by-products such as sulfur dioxide and nitric acid which enter the soil from acid rain. In most cases changes in soil pH, whether they are caused by natural processes or human activities, occur very slowly. This is the result of the tremendous buffering capacity (resistance to change in pH) of most mineral soils.

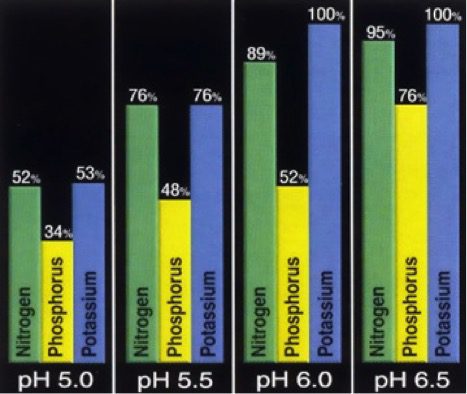
Effect of lime on fertilizer uptake in a lawn. As pH decreases so does the lawns ability to use the available nutrients in the soil.